Tirzepatide
发布时间:2025-03-06 10:20:35
共
0
家企业看过
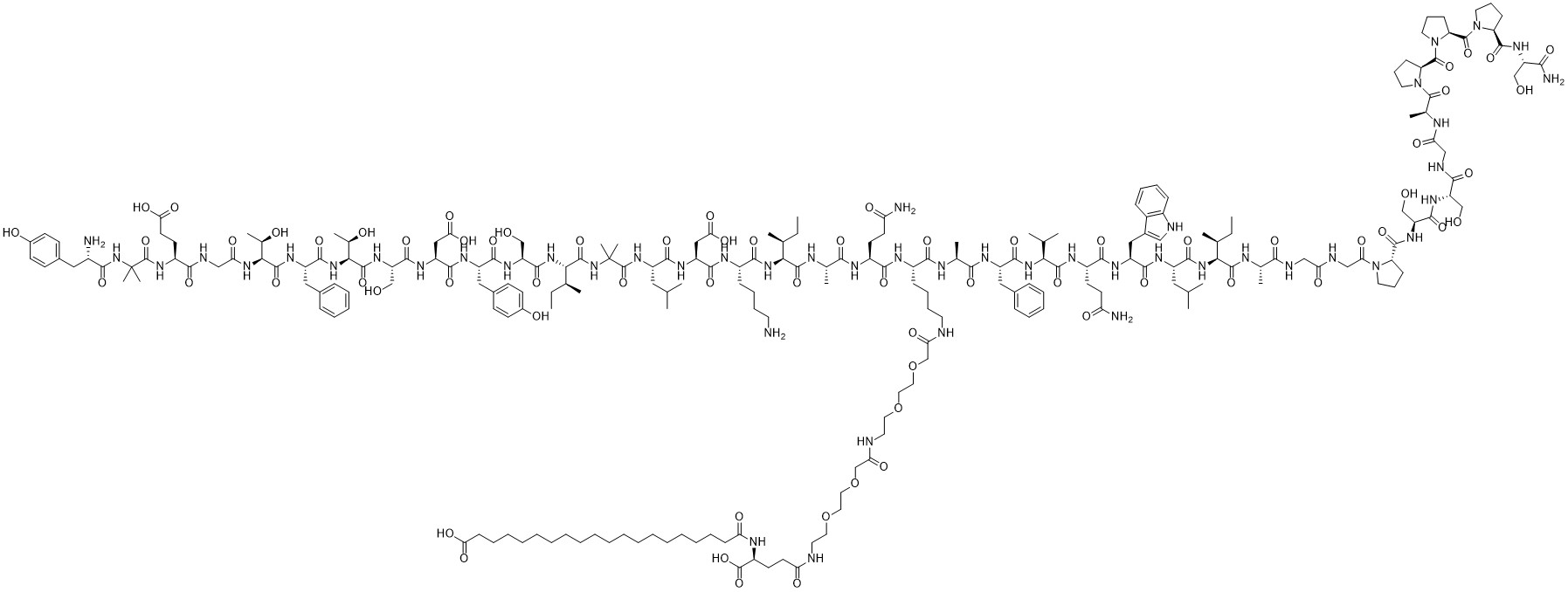
Tirzepatide,CAS:2023788-19-2,MW:4813.53,MF:C225H348N48O68
Tyr-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Aib-Leu-Asp-Lys-Ile-Ala-Gln-Lys(AEEA-AEEA-γ-Glu-Eicosanedioic acid)-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
Tirzepatide, a weekly double agonist of glucose dependent insulin stimulating peptide (GIP, also known as gastric inhibitory peptide) receptor and pancreatic peptide-1 (GLP-1) receptor developed by Lilly. In May 2022, Tirzepatide, as a "first in class" drug, was approved by the US FDA for marketing. It is the first new generation diabetes drug launched in the past decade. Tilposide defeated Smeglutide in phase III clinical head-on trials and is bound to become a strong competitor in the field of hypoglycemic and weight loss.
no cache
Processed in 0.491017 Second.
 For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust


