Difelikefalin
发布时间:2025-03-10 13:35:14
共
0
家企业看过
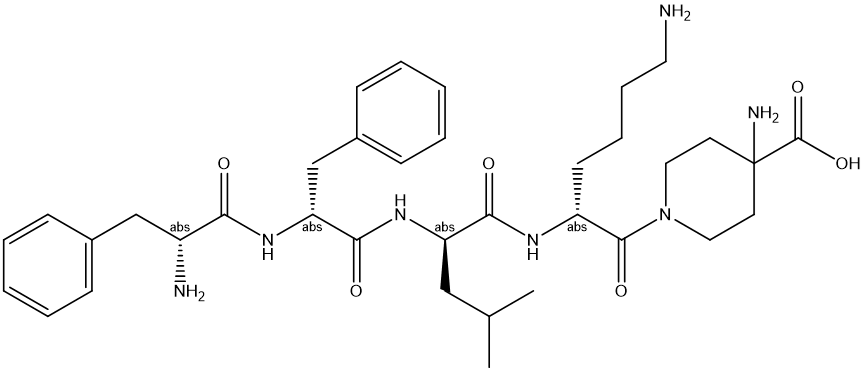
Sequence:1-(D-Phe-D-Phe-D-Leu-D-Lys)-4-aminopiperidine-4-carboxylic acid
MF: C36H53N7O6
MW: 679.86
CAS:1024829-44-4(Difelikefalin Acetate)
Chronic kidney disease associated itching (CKD-aP) is a refractory systemic itching disease that occurs frequently and with high intensity in patients with chronic kidney disease undergoing dialysis. There have also been reports of itching in stage III-V CKD patients without dialysis. Comprehensive, longitudinal, and multinational studies estimate that the weighted prevalence of CKD-aP in end-stage renal disease (ESRD) patients is approximately 40%, with approximately 25% of patients reporting severe itching.
On August 23, 2021, Cara Therapeutics and Vifor Pharma announced that the Food and Drug Administration (FDA) approved Korsuva (difelikefalin) injection for the treatment of moderate to severe itching related to chronic kidney disease in adults undergoing hemodialysis. Korsuva injection is a first in class κ Opioid receptor (KOR) agonists act on the peripheral nervous system of the human body. Previously, the FDA had granted Korsuva breakthrough drug qualification (BTD) for the treatment of this indication. Cara Therapeutics has requested the FDA to prioritize the review of new drug applications (NDAs).
no cache
Processed in 0.491017 Second.
 For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust
For many years, the original intention remains unchanged, just to do one thing well, the company always believes that professional first can cast the value of the brand. You are worthy of trust


